Hip replacement surgery is a targeted procedure used to treat arthritis due to wear and tear, bringing new life to many patients who endure chronic pain and loss of mobility.In 2020, approximately 10% and 20% of all primary and revision total hip replacements performed in the United States, respectively, used the dual-action molar cup (DM) because, compared to the traditional single-action joint DM offers greater range of motion and better joint stability and has been shown to be effective in reducing the risk of dislocation and instability in young and active patients, but there is currently no method to monitor DM motion in an in vitro simulated environment.
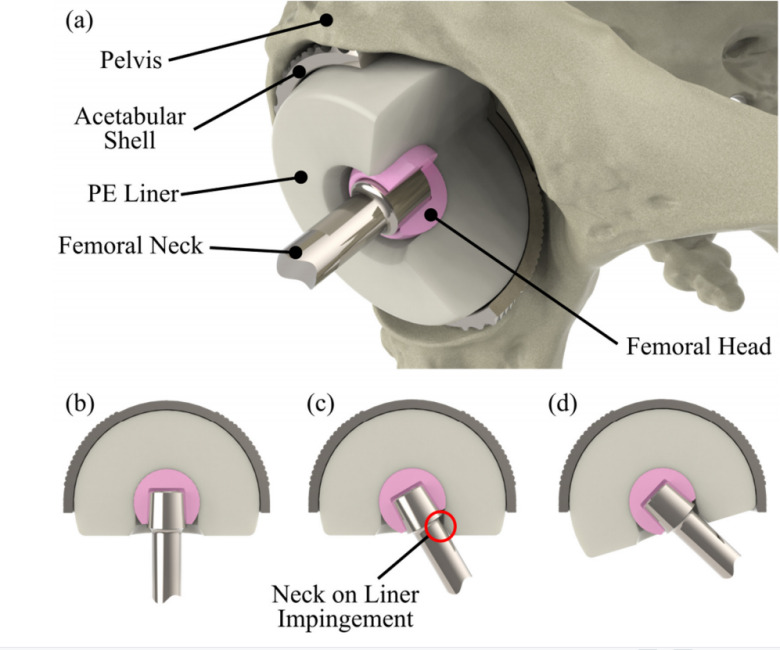
The DM consists of an acetabular shell, polyethylene liner, ball head and prosthetic stem. When the patient moves, the stem may hit the PE liner, which can cause the PE liner to loosen its connection to the acetabular shell as the motion increases. To monitor the movement of the liner, Matthew Peter Shuttleworth's research team from the UK designed a customized IMU (Inertial Measurement Unit) based tracking system.
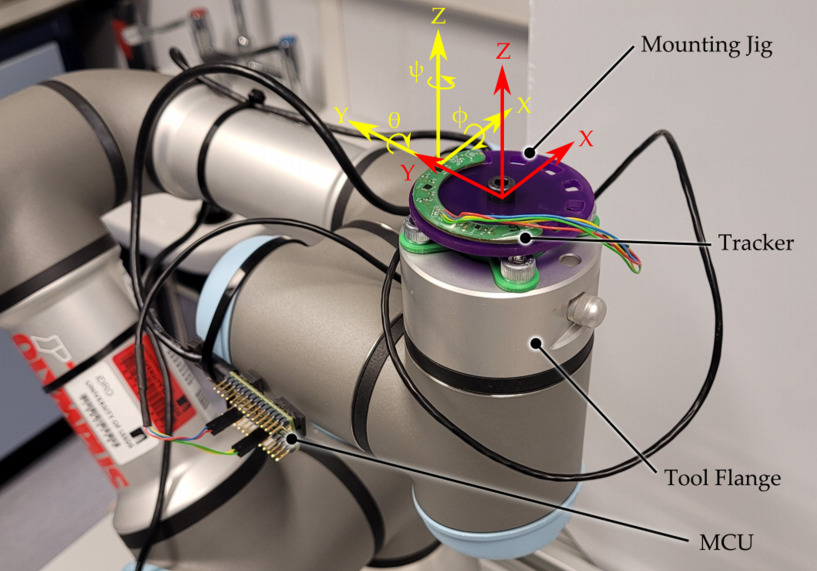
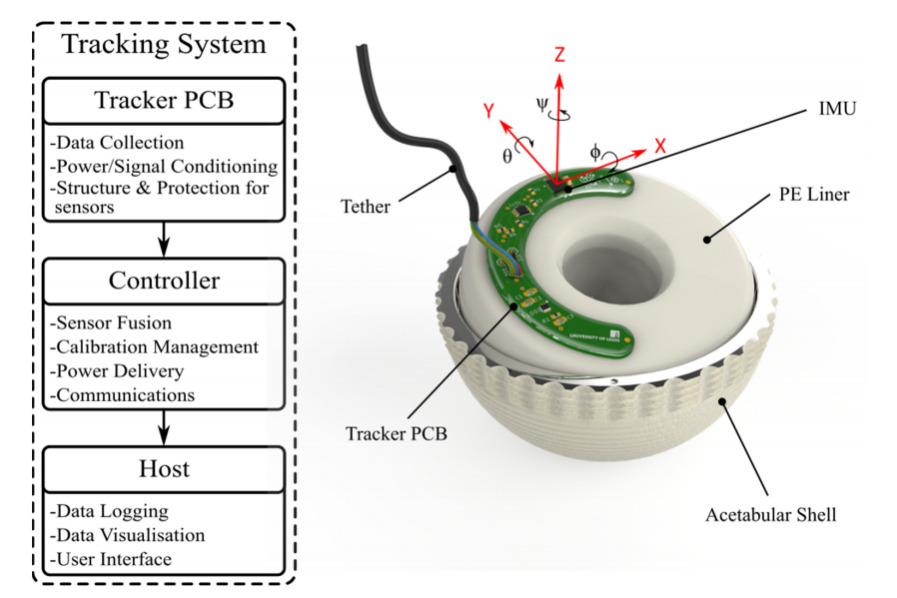
The tracking system consists of three modules: a tracking PCB circuit board coated with waterproof epoxy resin, a controller and a host computer. The system is mounted on the DM without affecting the bearing surface and the movement of the entire implant, and can be immersed in lubricating fluid to maintain normal operation, while being resistant to electromagnetic interference without excessive sensor drift, allowing accurate observation of the DM's movement.
After calibration of the IMU, experimental validation was performed under ideal conditions and in vitro in two environments.
Ideal conditions:
Experiments were conducted on the robot arm to evaluate the tracker performance by comparing the difference between the tracker data and the robot arm data. The results show that the average error value between the robot arm and the tracker is 0.2.
Under conditions of lubricant and magnetic interference outside the body:
Replicated experiments were conducted using phosphate buffer salt solution as the lubricant and solenoids as the magnetic interference device, and with and without solenoids installed. When the solenoid was not installed, the results showed greater errors compared to the ideal conditions; however, when the solenoid was used, the results were consistent with the ideal conditions experimental results.
This study is the first to successfully demonstrate the application of IMU to monitor the motion of a dual-action molar cup hip implant without a camera screen. As IMU products are innovated and algorithms optimized, we will be able to gain insight into the long-term function and potential failure modes of the DM, which in turn will help healthcare professionals with implant design, material selection, and surgical technique improvements to help hip patients better regain motion.
[Shuttleworth, Matthew Peter, et al. "Inertial Tracking System for Monitoring Dual Mobility Hip Implants In Vitro." Sensors 23.2 (2023): 904.]